Acute toxicity study of an aqueous extract of Ficus racemosa Linn. Acute toxicity study of an aqueous extract of Ficus racemosa Linn.
bark in albino mice - by Jaykaran, P Bhardwaj, N Kantharia, P Yadav, A Panwar
a) Abstract b) Introduction
c) Material And Methods
d) Acute Toxicity Study
e) Parameters For Observations |
f ) Observation & Results
g) Discussion
h) Conclusion
i) References
|
 |
|
| It is presumed that ayurvedic drugs have lesser side effects as compared to allopathic drugs. One of such herbs “ Ficus racemosa” has been mentioned for therapy of disorders like Diabetes, acidity, asthma, dysentery, menorrhagia, glandular enlargement, sore throat etc. However no references are available regarding the toxicity study of this herb. So we confined our work on acute toxicity studies of aqueous extract of Ficus racemosa Linn. bark in albino mice. Albino mice of either sex were divided into four groups 1st group given plain water and 2nd , 3rd , 4th given 100,300 and 1000mg of aqueous extract of herb per 100 gm body weight in single dose. After 72 hour of dose blood sample taken to determine haemoglobin, RBC count, WBC count, blood urea, blood glucose, serum Creatinine, serum cholesterol, S.G.P.T and S.G.O.T. Gross and histological examination done by veterinary pathologist. Result indicated that aqueous extract of Ficus racemosa did not have lethal effect upon 100 times of the therapeutic dose in albino mice. Although not dose dependent, this extract produced significant abnormality in liver and kidney. Fatty changes occurred in the kidneys. S.G.P.T level increased markedly. Fasting blood sugar continued to show inconsistent tendency toward hypoglycemia. |
Back to Top  |
|
 |
 |
|
| In the recent years, attention has been focused at the traditional (Herbal) way of therapy. It is presumed that Ayurvedic Medicines (drugs), which is popular in our country, have lesser side effects as compared to allopathic drugs.
One of such herbs “Ficus racemosa” has been mentioned for therapy of various disorders like: diabetes, amlapitta (acidity), asthma, dysentery, menorrhagia, glandular enlargement, sore throat etc. in an ancient Literature of Ayurveda12 .
Various effects of Ficus racemosa have been also studied using modern scientific methods i.e. anti-ulcer 3 & anti-secretory 4 activity, anti-protozoal 5 and anti-fungal 6 activity, hypotensive and vasodilator activity 7 , wound healing and anti-inflammatory activity 8 , hepato-protective 9 10 and antidiabetic 11 properties also have been reported.
However, no work has been carried out regarding toxicity studies of Ficus racemosa Linn. (Bark). For this reason we confined our work on “Acute toxicity studies of an aqueous extract of Ficus-racemosa Linn. bark in albino mice.” |
Back to Top  |
|
 |
 |
|
| Drug : The plant material ( bark of Ficus racemosa ) was collected in March – April 99, from local sources and identified with the help of a pharmacognosist of Gujarat Ayurvedic University, Jamnagar. The bark was dried for 2 to 3 weeks at room temperature and then powdered in the grinder. Then finally the aqueous extract of the bark powder was prepared 12 . Studies carried out in our department earlier have shown hypoglycemic activity in the dose range of 20-80 mg/kg and anti-ulcer activity in the dose range of 40- 200 mg/kg of body weight. Hence 100 mg/ kg is taken as rough estimate of mean of above dose ranges. 34
Animals : The Swiss albino mice weighing 30 to 60 grams of either sex respectively were produced from Laboratory Animals Resource Section, Gujarat Ayurvedic University, Jamnagar and they were fed with standard pelleted laboratory diet and ordinary tap water. The study was designed to evaluate acute toxicity of the drug. |
Back to Top  |
|
 |
 |
|
| For evaluating acute toxicity, albino mice of either sex were housed; four animals per cage for one week to acclimatized and were provided food and water ad - libitum. Then the mice were housed one per cage for 5 days for food training i.e. ( they were provided food only for 6 hrs. / day ).
Then the mice were divided into 4 groups: (6 mice in each group) Allocation was done in randomized fashion. Average food intake for three days was measured in all animals, before starting the experiment. First group received plain water only, and was used as control. Group 2, 3 and 4 were treated orally with 100 mg, 300 mg and 1000 mg per 100 g of the Body-weight single dose of aqueous extract of Ficus racemosa bark respectively. This amounts exposed the animals to 10, 30 and 100 times the therapeutic dose of the drug. The monitoring of the parameters commenced immediately after administrating the drug. Animals were observed at 0 hr, 1 hr, 2 hrs, 4 hrs, 6 hrs, 8 hrs, 24 hrs and 72 hrs.
|
Back to Top  |
|
 |
 |
Parameters For Observations |
|
Mortality of animals, Motor activity, Tremors, Convulsions, Posture, Spasticity, Opisthotonicity, Ataxia, Righting reflex, Sensations, Pilo-erection, Ptosis, Lacrymation, Exopthalmos, Salivation, Diarrhoea, Writhing, Skin color and Respiratory rate. Daily food intake & weight of the animals were also recorded. Three days (72 hours) after the oral administration of the drug, blood was drawn from each of the animal by cardiac puncture to determine: Hemoglobin, R.B.C. count, W.B.C. count, Blood Urea, Blood Glucose, Serum Creatinin, Serum Cholesterol and S.G.P.T. ( Blood chemistry was carried out in the Pathology Laboratory using semi auto analyzer ). Then the animals were sacrificed, their Liver & Kidney dissected out and their Gross and Histo pathological examinations were done by a veterinary pathologist who was not knowing the distribution of animal in study group and control group 12131415 .
* Data was analysed by using student ‘t’ test ( unpaired ) ‘p’ value of < 0.05 was considered as statistical significant16 .
|
Back to Top  |
|
 |
 |
|
Acute toxicity study of an aqueous extract of Ficus racemosa Linn. (Bark)
Figure 1
Not a single animal died even after 100 times the normal (Therapeutic) dose. We could not expose the animal to the doses above 100 times the therapeutic dose because above that, the powdered extract could not be dissolved in water and solution became too thick to handle.
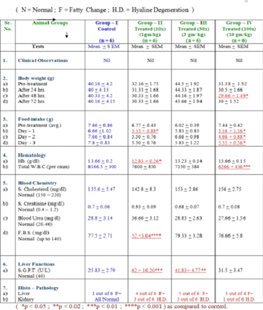
No significant clinical findings were noted in any of the group. Body weight did not change significantly in any treated group except group IV, day 2 nd as compared to control group. Food intake was significantly lowered on day 1 in group II (10 x) and group IV (100 x) and continued to be lowered on day 2 and 3 in group- IV.
Although Hb concentration turned to be significantly different in group II ( 10 x), no other group showed any changes. And even in group II, the difference dose not have any clinical significance. Total W.B.C. (per cmm) shows significant difference in group IV as compared to control group statistically.
We found that Serum cholesterol remained unchanged, while F.B.S. was significantly lower in-group II. There was no effect on F.B.S. in group treated with higher doses (30x and 100x). In previous two studies also, consistency in hypoglycemic effect and dose dependency were lacking 11 .
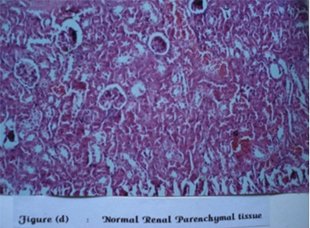
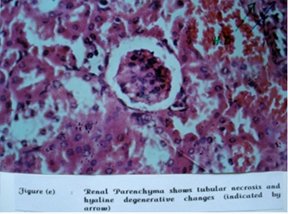
Serum creatinine and blood urea remained unchanged during acute toxicity study. However, on histopathological examination seven out of total eighteen animals had developed hyaline degenerative changes as compare to none in the control group.
Liver function was significantly altered and S.G.P.T. showed significant rise as compared to normal (control).
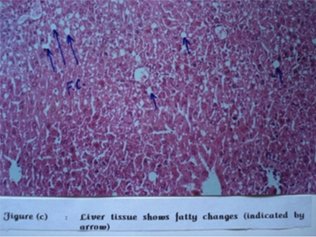
Histo-pathological examination further established possible acute hepato-toxicity. 12 out of 18 animals developed fatty changes to a greater or lesser extent. In first treated group fatty changes were observed in 2nd , 3rd , 5th and 6th mice. In second treated group it was observed in 1st , 2nd , 3rd , 4th and 5th mice. In third group fatty changes were observed in 1st , 4 th and 6 th mice.
------------------------------------------------------------------------------------------------------------------
Figure 2
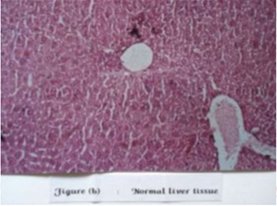
Figure 1: Normal liver tissue
------------------------------------------------------------------------------------------------------------------
Figure 3
Figure 2: Liver tissue shows fatty changes (indicated by arrow)
Hyaline degeneration of the kidney section was observed in 2 nd , 3 rd and 5 th mice of first treated group (1 gm/kg). In second treated group (3 gm/kg) this was observed in 1 st , 2 nd and 5 th mice. In third group (10gm/kg) hyaline degeneration was observed in 6 th mice
------------------------------------------------------------------------------------------------------------------
Figure 4
Figure 3: Normal Renal Parenchymal tissue
------------------------------------------------------------------------------------------------------------------
Figure 5
Figure4: Renal Parenchyma shows tubular necrosis and hyaline degenerative changes
|
Back to Top  |
|
 |
 |
|
When a drug is used, it is expected to benefit the recipient. At the same time, it is a fact that there is no drug, which is totally free from harmful effects. And therefore when any drug is used, it is necessary to consider not only the beneficial effect but also the harmful effects. A drug can be used only after a careful weighing of pros and cons, the benefit: risk ration. When this ratio is favorable to the patient in a given situation, we can use the drug.
This is true for any drug, whether it is synthetic chemical compound or a plant product. All the drugs have to pass through stringent toxicity testing, before approval can be granted for there use in general population.
In recent years there is increasing trend for using alternative system of medicine. It is argued, that such drugs are not only effective but also very safe as compared to allopathic drugs for the similar indications. The claim that natural plant product are safe should be accepted only after the plant product passes through toxicity testing using modern scientific methods.
Acute toxicity studies in animals are of value in predicting potential toxic effects of a chemical in human beings exposed to near fatal doses. From these studies nature of acute response in man may be anticipated. It also gives rough idea about the organ system involvement.
Ficus racemosa (Cluster Fig, Gular) is habitat of most of the parts of India. 2 Its roots, bark, fruits, milky juice, leaves and gall on leaves have been used for medicinal purpose. 12 Chemically it contains tannins, wax, caoutchouc, ash, silica and phosphoric acid. 2 On chemical analysis it found to contain water-13.6%, albuminoids- 7.4%, oil – 5.6%, carbohydrates- 49.0%, fibres- 17.9% and ash – 6.5%. alcoholic extract contains a trace of soluble tannin.
Use of alcohol extract of stem bark of Ficus racemosa mentioned in various studies. 58 but work is also carried on aqueous extract. Patel and Vasavada (1985) 3 and Kharod B.N. (1993) 4 studied antiulcer activity and anti secretory activity of aqueous extract of Ficus racemosa bark.
Anti-secretory activity of the drug was demonstrated in pyloric ligated rat model of ulcer. Their finding concludes that the aqueous extract of Ficus racemosa Linn. bark at the dose level of 20 mg/100 gm body weight in albino rats produces antiulcer and anti-secretory activity.In the Study of effect of aqueous extract of Ficus racemosa Linn. bark on experimental models of wounds and on acute and chronic inflammatory models (albino rat) was carried out by Mapara D.S. (1999) 8 and result are suggestive that the drug has got wound healing property and anti-inflammatory activity. 8
Similarly in another study, it was found that aqueous extract of bark of Ficus racemosa Linn produced hypoglycemic effect in diabetic as well as non diabetic animals at different doses, 20, 40, 80 mg/kg in albino rats. 11
In a study done by Krishnamurthi et al (2007), antihyperglycemic and antilipidperoxidative effects of aqueous and ethanolic extract of Ficus racemosa Linn bark were measured in alloxan induced diabetes rat. Oral administration of aqueous and ethanolic extract in the dose of 400 mg and 300 mg per kg were given respectively. Aqueous extract showed better hypoglycemic activity than glibenclamide and ethanolic extract’s effect was comparable to glibenclamide. Increased levels of blood glucose and decreased plasma insulin in diabetic rats are either due to decreased utilization or defect in the activities of carbohydrate metabolizing enzymes. Ficus racemosa stimulate more insulin secretion from surviving pancreatic beta cells or promote more glucose utilization in peripheral tissue. 17
This is the first study regarding the acute toxicity of this plant as far as out knowledge goes so we were not able to compare our data with other studies.Our study haS some limitations. There was difference in mean body weight between the groups. It was purely because of chance as stratified randomization was done during formation of these groups.
|
Back to Top  |
|
 |
 |
|
Aqueous extract of Ficus - racemosa Linn. bark did not have any Lethal effect up to 100 times of the therapeutic dose in the Albino mice.
Although not dose dependent, this extract produced significant abnormality in liver and kidneys during acute toxicity studies. Fatty changes occurred in liver and hyaline degenerative changes occurred in the kidneys. S.G.P.T. levels, increased markedly while serum creatinine and blood urea remained unchanged. Fasting blood sugar continued to show inconsistent tendency towards hypoglycemia.
This study is not a complete toxicity study; it emphasizes the need for carrying out toxicity studies even in natural plant (Herbal) products and drug of indigenous medicinal system.
|
Back to Top  |
|
 |
 |
|
1. Kirtikar KR, Major Basu BD, Basu LM. Indian Medicinal Plants. Second edition. Allahabad, 1953; 3: 2327.
2. Nadkarni AK, Indian Materia Medica. 3rd edition. Panvel. Dhootpapeshwar Prakashan Ltd., 1954; 3: 548.
3. Patel SM, Vasavada SA, A study on Ficus racemosa Linn. Anti-ulcer activity (Part – I) Bulletin of Medica Ethano Botanical Research. No. 1, 1986; 6: 17- 27.
4. Kharod BN, The effect of an aqueous extract of Ficus racemosa Linn. bark on gastric and duodenal ulcers. Dissertation. M.P.Shah Medical College. Jamnagar, 1993.
5. Dhar ML, Dhar MM, Dhavan BN, Malhotra BN and Ray C. Screening of Indian Plants for Biological activity – 1, Indian Journal Experimental Biology 1968 ; 6 : 232.
6. Deraniyagola SA, Wijesundera RLC, Weerasena. Journal of the National Science Council of Srilanka, 1998; 26: 1, 19-26.
7. Trivedi CP, Shinde S and Sharma RC. Studies of the preliminary Phytochemical and Pharmacological activity of Ficus racemosa (Gular). Indian Journal of Medical Research, 1969; 57: 1070.
8. Mapara DS, Effect of an aqueous extract of Ficus racemosa Linn. bark on wound healing and anti-inflammatory activity in albino rats. Dissertation. M.P.S.M. College, Jamnagar, 1996.
9. Mandal SC, Maity TK, Das J, Pal M and Saha BP. Calcutta. Phyto-therapy Research, 1999; 13: 5, 430 – 432.
10. Mandal SC, Maity TK, Das J, Pal M and Saha BP. Calcutta. Natural Product Science, 1998; 4: 3, 174 – 179.
11. Halavadiya L. Study of anti-diabetic activity of an aqueous extract of Ficus raceosa Linn. bark in albino rats. Dissertation, 2000.
12. Boyd EM, Predictive Toxicometrics. England. Scientechina (Publishers) Ltd. 1972.
13. Plwa GL, Smith RP. General Principles of Toxicology. Munson PL, In: Principle of Pharmacology. Basic Concept and Clinical Application. Newyork. Chapman and Hall, 1994-95: 1538 – 39.
14. Lehman AJ, The intent of the pre – clinical assessment of new drugs. Lecture of joint symposium on evaluation of new drugs. AMA sect. Expr. Med. And Therapy and Society of Toxicology. Atlantic City N.J. June 17 / 1963.
15. Turner RA. Screening Methods in Pharmacology. 1st edition, U.S.A. ( New York ) Academic Press, 1965.
16. Ghosh MN. Fundamentals of Experimental Pharmacology. 2nd edition Calcutta. Scientific Book Agency, 1984.
17. Vasudevan K et al. Antihyperglycemic and antilipidperoxidative effects of Ficus racemosa (Linn.) bark extract in alloxan induced diabetic rats 2007; Journal of Medical Science: 7:330-338.
|
Back to Top  |
|
 |
|